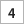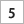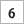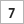The team should place each marked piece so that “U-235” is showing. This represents Uranium-235, which emits a series of particles from the nucleus as it decays to Lead-207 (Pb- 207). When each team is ready with the 128 pieces all showing “U-235”, a timed two-minute interval should start. During that time each team turns over half of the U-235 pieces so that they now show Pb-207.
These thick layers alternate with thin, clay-rich layers deposited during the winter. The resulting layers, calledvarves, give scientists clues about past climate conditions. For example, an especially warm summer might result in a very thick layer of sediment deposited from the melting glacier. Thinner varves can indicate colder summers, because the glacier doesn’t melt as much and carry as much sediment into the lake. Explain how the decay of radioactive materials helps to establish the age of an object. • Their heaviness allows them to be easily separated from lighter minerals using a shake table.
The presence or absence of zircon grains with ages approximating the time of accumulation of the host sediment likely reflects the proximity of the basin to a plate margin. The overall spread of ages is a function of the nature of the source and the area of the distributive province, with large hinterlands more likely to provide a variety of source ages. Older source regions provide an episodic, rather than a continuous, age distribution (Hawkesworth et al., 2009). It has a magnetic north and south pole and its magnetic field is everywhere .
1. Sample preparation
The peaks showing the most numerously computed ages are compared with possible igneous or metamorphic sources for the sediment. Since zircon geochronology is so precise, sources can often be pinpointed to a particular body of magma. This is particularly helpful in determining the courses of ancient drainage systems. Ordovician-Silurian ages (data ranging from 494±14 to 413±9 Ma) have been recorded in augen gneisses, fine-grained gogaper.com leucocratic gneisses and granulite-facies metabasites from Calabria [16-18, 7, 24]. In these rock-types the Ordovician-Silurian ages represent clusters connected to a recrystallization event being the protoliths Neoproterozoic-Cambrian in origin. These ages were measured on cores displaying irregular and patchy microstructures sometimes strongly luminescent (Fig. 7 a-b) or on overgrowths surrounding older cores (Fig. 7 c-d).
1000 Ma, 700–500 Ma, 375 Ma, and 260–200 Ma zircon age populations (Elliot and Fanning, 2008; Goodge and Fanning, 2010; Elsner et al., 2013; Elliot et al., 2015, 2017). Example for the visual comparison of detrital zircon age distributions (in million years; Ma) of samples for provenance analysis via detecting similarities or differences in their zircon age populations (Plots Chiara Költringer). For example, if the measured abundance of 14C and 14N in a bone are equal, one half-life has passed and the bone is 5,730 years old (an amount equal to the half-life of 14C).
Absolute Ages of Rocks
Crystals may form multiple crystal layers, with each layer recording the isotopic age of an event, thus tracing the progress of the several metamorphic events. Where sedimentary layers were deposited on top of crystalline rocks . If a 1500-million-year-old rock is disturbed to create a discordia, then is undisturbed for another billion years, the whole discordia line will migrate along the curve of the concordia, always pointing to the age of the disturbance.
The main limitation is that it only works on certain igneous rocks as most rocks have insufficient Re and Os or lack evolution of the isotopes. This technique is good for iron meteorites and the mineral molybdenite. Some do not change with time and form stable isotopes (i.e. those that form during chemical reactions without breaking down). The unstable or more commonly known radioactive isotopes break down by radioactive decay into other isotopes.
Data & Figures
Understanding the ages of related fossil species helps scientists piece together the evolutionary history of a group of organisms. If an atom decays by losing a beta particle, it loses just one electron. The discovery of radioactive materials did more than disprove Thomson’s estimate of Earth’s age.
Supplementary data
Using logs recovered from old buildings and ancient ruins, scientists have been able to compare tree rings to create a continuous record of tree rings over the past 2,000 years. This tree ring record has proven extremely useful in creating a record of climate change, and in finding the age of ancient structures. According to the principle of original horizontality, these strata must have been deposited horizontally and then titled vertically after they were deposited. In addition to being tilted horizontally, the layers have been faulted . Applying the principle of cross-cutting relationships, this fault that offsets the layers of rock must have occurred after the strata were deposited. Just as when they were deposited, the strata are mostly horizontal .
In case of trace element analysis we used NIST610 as primary reference material and zircon for quality control, and an in-house synthetic reference material to correct the Nb concentrations in some sessions. We analyzed the samples in 12 sessions and in case of 9 sessions U-Pb isotopes and trace elements were determined simultaneously . With some variations between sessions the target elements were Si, Zr, REE, Y, Hf, P, Nb, Ta, U, Th, Ti , and either Al, Rb, Ba, Ca, Fe or Al, Sr were measured for monitoring glass, apatite and iron oxide inclusions. Si (15.2 wt% in Zircon) was used as internal standard for data reduction done by IOLITE. Spot compositions that has larger values than the detection limit for Al, Fe, Ca were discarded from the database.
The amount of 14C present and the known rate of decay of 14C and the equilibrium value gives the length of time elapsed since the death of the organism. Some techniques place the sample in a nuclear reactor first to excite the isotopes present, then measure these isotopes using a mass spectrometer (such as in the argon-argon scheme). Others place mineral grains under a special microscope, firing a laser beam at the grains which ionises the mineral and releases the isotopes. The isotopes are then measured within the same machine by an attached mass spectrometer . The discovery of radioactive materials did more than disprove Thomson’s estimate of Earth’s age. To understand how this is done, it is necessary to review some facts about atoms.
In this case, fossils can be useful tools for understanding the relative ages of rocks. Each fossil species reflects a unique period of time in Earth’s history. The principle of faunal succession states that different fossil species always appear and disappear in the same order, and that once a fossil species goes extinct, it disappears and cannot reappear in younger rocks . Sometimes sedimentary rocks are disturbed by events, such as fault movements, that cut across layers after the rocks were deposited. The principle states that any geologic features that cut across strata must have formed after the rocks they cut through . Uranium-lead dating is usually performed on crystals of the mineral zircon (Figure 11.26).
The team should place each marked piece so that “U-235” is showing. This represents Uranium-235, which emits a series of particles from the nucleus as it decays to Lead-207 (Pb- 207). When each team is ready with the 128 pieces all showing “U-235”, a timed two-minute interval should start. During that time each team turns over half of the U-235 pieces so that they now show Pb-207.
These thick layers alternate with thin, clay-rich layers deposited during the winter. The resulting layers, calledvarves, give scientists clues about past climate conditions. For example, an especially warm summer might result in a very thick layer of sediment deposited from the melting glacier. Thinner varves can indicate colder summers, because the glacier doesn’t melt as much and carry as much sediment into the lake. Explain how the decay of radioactive materials helps to establish the age of an object. • Their heaviness allows them to be easily separated from lighter minerals using a shake table.
The presence or absence of zircon grains with ages approximating the time of accumulation of the host sediment likely reflects the proximity of the basin to a plate margin. The overall spread of ages is a function of the nature of the source and the area of the distributive province, with large hinterlands more likely to provide a variety of source ages. Older source regions provide an episodic, rather than a continuous, age distribution (Hawkesworth et al., 2009). It has a magnetic north and south pole and its magnetic field is everywhere .
1. Sample preparation
The peaks showing the most numerously computed ages are compared with possible igneous or metamorphic sources for the sediment. Since zircon geochronology is so precise, sources can often be pinpointed to a particular body of magma. This is particularly helpful in determining the courses of ancient drainage systems. Ordovician-Silurian ages (data ranging from 494±14 to 413±9 Ma) have been recorded in augen gneisses, fine-grained gogaper.com leucocratic gneisses and granulite-facies metabasites from Calabria [16-18, 7, 24]. In these rock-types the Ordovician-Silurian ages represent clusters connected to a recrystallization event being the protoliths Neoproterozoic-Cambrian in origin. These ages were measured on cores displaying irregular and patchy microstructures sometimes strongly luminescent (Fig. 7 a-b) or on overgrowths surrounding older cores (Fig. 7 c-d).
1000 Ma, 700–500 Ma, 375 Ma, and 260–200 Ma zircon age populations (Elliot and Fanning, 2008; Goodge and Fanning, 2010; Elsner et al., 2013; Elliot et al., 2015, 2017). Example for the visual comparison of detrital zircon age distributions (in million years; Ma) of samples for provenance analysis via detecting similarities or differences in their zircon age populations (Plots Chiara Költringer). For example, if the measured abundance of 14C and 14N in a bone are equal, one half-life has passed and the bone is 5,730 years old (an amount equal to the half-life of 14C).
Absolute Ages of Rocks
Crystals may form multiple crystal layers, with each layer recording the isotopic age of an event, thus tracing the progress of the several metamorphic events. Where sedimentary layers were deposited on top of crystalline rocks . If a 1500-million-year-old rock is disturbed to create a discordia, then is undisturbed for another billion years, the whole discordia line will migrate along the curve of the concordia, always pointing to the age of the disturbance.
The main limitation is that it only works on certain igneous rocks as most rocks have insufficient Re and Os or lack evolution of the isotopes. This technique is good for iron meteorites and the mineral molybdenite. Some do not change with time and form stable isotopes (i.e. those that form during chemical reactions without breaking down). The unstable or more commonly known radioactive isotopes break down by radioactive decay into other isotopes.
Data & Figures
Understanding the ages of related fossil species helps scientists piece together the evolutionary history of a group of organisms. If an atom decays by losing a beta particle, it loses just one electron. The discovery of radioactive materials did more than disprove Thomson’s estimate of Earth’s age.
Supplementary data
Using logs recovered from old buildings and ancient ruins, scientists have been able to compare tree rings to create a continuous record of tree rings over the past 2,000 years. This tree ring record has proven extremely useful in creating a record of climate change, and in finding the age of ancient structures. According to the principle of original horizontality, these strata must have been deposited horizontally and then titled vertically after they were deposited. In addition to being tilted horizontally, the layers have been faulted . Applying the principle of cross-cutting relationships, this fault that offsets the layers of rock must have occurred after the strata were deposited. Just as when they were deposited, the strata are mostly horizontal .
In case of trace element analysis we used NIST610 as primary reference material and zircon for quality control, and an in-house synthetic reference material to correct the Nb concentrations in some sessions. We analyzed the samples in 12 sessions and in case of 9 sessions U-Pb isotopes and trace elements were determined simultaneously . With some variations between sessions the target elements were Si, Zr, REE, Y, Hf, P, Nb, Ta, U, Th, Ti , and either Al, Rb, Ba, Ca, Fe or Al, Sr were measured for monitoring glass, apatite and iron oxide inclusions. Si (15.2 wt% in Zircon) was used as internal standard for data reduction done by IOLITE. Spot compositions that has larger values than the detection limit for Al, Fe, Ca were discarded from the database.
The amount of 14C present and the known rate of decay of 14C and the equilibrium value gives the length of time elapsed since the death of the organism. Some techniques place the sample in a nuclear reactor first to excite the isotopes present, then measure these isotopes using a mass spectrometer (such as in the argon-argon scheme). Others place mineral grains under a special microscope, firing a laser beam at the grains which ionises the mineral and releases the isotopes. The isotopes are then measured within the same machine by an attached mass spectrometer . The discovery of radioactive materials did more than disprove Thomson’s estimate of Earth’s age. To understand how this is done, it is necessary to review some facts about atoms.
In this case, fossils can be useful tools for understanding the relative ages of rocks. Each fossil species reflects a unique period of time in Earth’s history. The principle of faunal succession states that different fossil species always appear and disappear in the same order, and that once a fossil species goes extinct, it disappears and cannot reappear in younger rocks . Sometimes sedimentary rocks are disturbed by events, such as fault movements, that cut across layers after the rocks were deposited. The principle states that any geologic features that cut across strata must have formed after the rocks they cut through . Uranium-lead dating is usually performed on crystals of the mineral zircon (Figure 11.26).